Immuno-metabolic dialogue in obesity and its comorbidities
Team 3 – INSERM U1011 – Lille University – CHU Lille – Institut Pasteur de Lille

Presentation
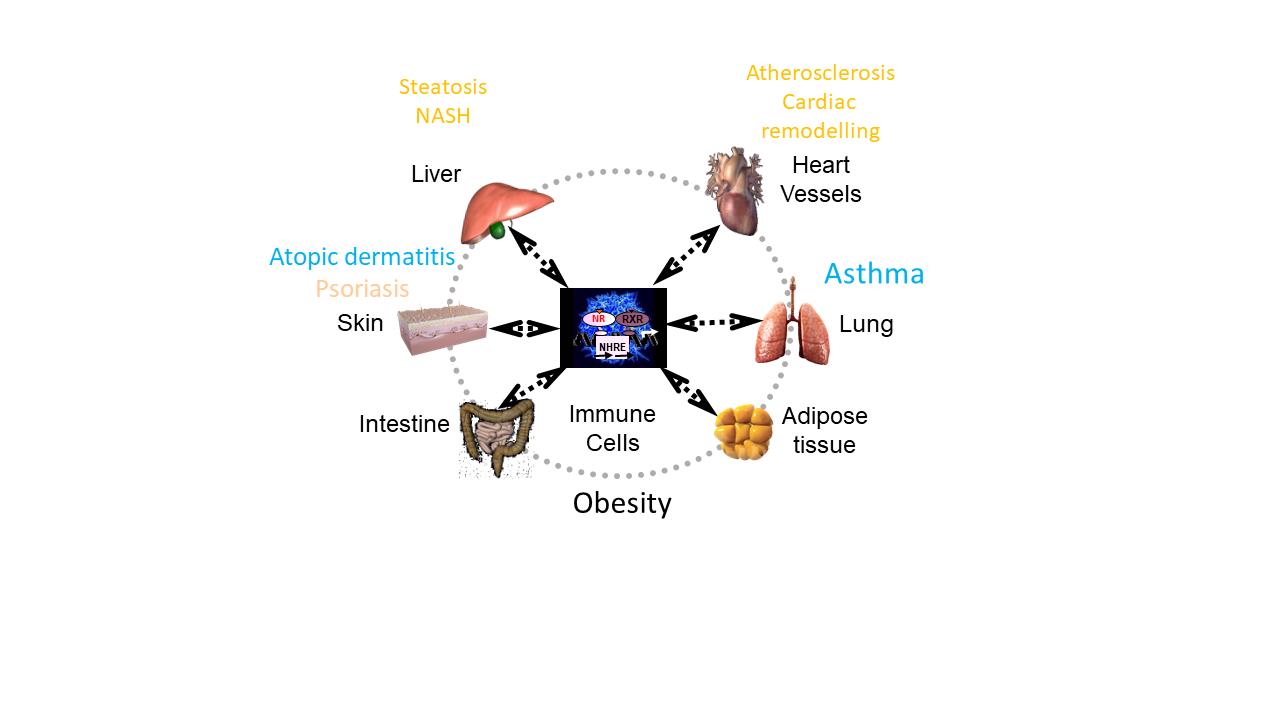
The immuno-inflammatory and metabolic systems are tightly linked, as revealed by the frequent co-morbidities between (auto)inflammatory or (auto)immune diseases (asthma, psoriasis…) and metabolic pathologies and their cardiovascular complications. Immune cells play an essential role in pathologies once considered as purely metabolic. Conversely, immune cells depend on metabolism for their energy supply. Therefore, metabolic pathologies have an impact on the metabolism of immune cells and thus on their functions. Nuclear receptors are transcriptional regulators that control immune and metabolic processes and are therapeutic targets of choice. Our research aims to identify and elucidate the keys to the immuno-metabolic dialogue, and in particular the contribution of nuclear receptors expressed by immune cells in different pathophysiological contexts related to metabolism (obesity, type 2 diabetes, atherosclerosis, NAFLD/NASH…) and its co-morbidities.
Highlights
- We discovered how fatty acids from the metabolic environment modulate the innate immune response through the triggering of a specific UPR-mediated Integrated Stress Response and and demonstrated their role in psoriasis exacerbation (Mogilenko et al., Cell, 2019).
- We identified a specific transcriptomic and cellular signature of the transition from benign steatosis to NASH in humans and in a preclinical model with dendritic cells cDC subsets and cytotoxic CD8 T lymphocytes as hallmarks (Haas, et al., Nature Metabolism, 2019).
- We demonstrated the key regulatory role of the nuclear receptors PPARa and d in sepsis and anaphylaxis (Wawrzyniak et al., JACI, 2015, Paumelle et al., Hepatol, 2019).
- We discovered that Innate Lymphoid Cells as essential for aggravation of allergic asthma by obesity (Everaere et al., JACI, 2016).
- We unraveled the key role of CX3CL1-CX3CR1 axis for inflammatory CD4 T cell function in allergic asthma and atopic dermatitis (Mionnet et al., Nature Medicine, 2010, Staumont-Salle et al., Exp. Med, 2014).
- We established the Metabolic ImmunoPhenotyping Platform (PIM, U1011-EGID) equipped with state of the art flow and mass cytometrs (Aria X20, Influx, Helios).
- We established the functional exploration of lung in mouse by Invasive Plethysmography (Institut Pasteur de Lille).
Membres
David DOMBROWICZ
Research director, team leader
ORCID number : 0000-0002-0485-8923
Laurent L’HOMME
Post-doc
Johanna HOOGERLAND
Post-doc
Artemii NIKITIN
Post-doc
Sébastien FLEURY
Engineer, Inserm
Marie-Laure JOSEPH
Technician, IPL
Olivier MOLENDI-COSTE
Engineer, Inserm
Samuel PIC
Engineer, Univ Lille
Laurent PINEAU
Engineer, IPL
Sandrine QUEMENER
Engineer, Univ Lille
Valentine GUINOT
PhD student
Pelin SERMIKLI
Post-doc
Marie BICHAREL-LECOMTE
Master 2
Publications
Mogilenko, D.A., Haas, J.T., L’Homme, L., Fleury, S., Quemener, S., Levavasseur, M., Becquart, C., Wartelle, J., Bogomolova, A., Pineau, L., Molendi-Coste, O., Lancel, S., Dehondt, H., Gheeraert,C. Melchior, A., Dewas, C., Nikitin, A., Pic, S., Rabhi, N., Annicotte, J.-S, Oyadomari, S., Velasco-Hernandez, T., Cammenga, J., Foretz, M., Viollet, B., Vukovic, M., Villacreces, A., Kranc, K., Carmeliet, P., Marot, G., Boulter, A., Tavernier, S., Berod, L., Longhi, M. P., Paget, C., Janssens, S., Staumont-Sallé, D§., Aksoy, A§., Staels, B§., and D. Dombrowicz (2019).
Metabolic and Innate Immune Cues Merge into a Specific Inflammatory Response via the UPR.
Cell 177, 1201-1216 e1219l (avec éditorial). Avancée de l’Inserm 2019
Haas, J.T. !§, Vonghia§, L., Mogilenko§, D.A., Verrijken, A., Molendi-Coste, O., Fleury, S., Deprince, A., Nikitin, A., Woitrain, E., Ducrocq-Geoffroy, L, Pic, S., Derudas, B., Dehondt, H., Gheeraert, C., Van Gaal, L., Driessen, A., Lefebvre, P., Staels B*, Francque S* and D. Dombrowicz* (2019).
Transcriptional network analysis implicates altered hepatic immune function in NASH development and resolution.
Nature Metabolism 1, 604-614.
Paumelle, R., Haas, J.T., Hennuyer, N., Bauge, E., Deleye, Y., Mesotten, D., Langouche, L., Vanhoutte, J., Cudejko, C., Wouters, K., Hannou SA, Legry V, Lancel S, Lalloyer F, Polizzi A, Smati S, Gourdy P, Vallez E, Bouchaert E, Derudas B, Dehondt H, Gheeraert C, Fleury S, Tailleux A, Montagner A, Wahli W, Van Den Berghe G, Guillou H, Dombrowicz*, D. and B. Staels* (2019).
Hepatic PPARa is critical in the metabolic adaptation to sepsis.
J Hepatol 70, 963-973.
Everaere, L., S. Ait-Yahia, O. Molendi-Coste, H. Vorng, S. Quemener, P. LeVu, S. Fleury, E. Bouchaert, Y. Fan, C. Duez, P. de Nadai, B. Staels, D. Dombrowicz*, and A. Tsicopoulos*. 2016.
Innate lymphoid cells contribute to allergic airway disease exacerbation by obesity.
J Allergy Clin Immunol . 138,1309-1318 e11 (avec editorial).
Julia, V., Macia, L., and Dombrowicz, D. (2015).
The impact of diet on asthma and allergic diseases.
Nat Rev Immunol 15, 308-322.
Keywords
Inflammation ; Immuno-metabolism ; Immune system ; Metabolic and cardio-vascular diseases ; NAFLD/NASH ; Bile acids ; Mass cytometry ; Nuclear receptors ; Psoriasis ; Allergy