Tropical biomes and immune-pathophysiology
INSERM U1019 – CNRS UMR9017 – Lille University – CHU Lille & Guyane University – Institut Pasteur de Lille
Presentation
The general objective of the team’s research project is to evaluate the complex nature of immune mechanisms induced during protozoan parasite (co)infections. In the high endemic areas of the Amazonian ecosystem of the French Guiana concomitant infections by Plasmodium, Leishmania and Toxoplasma cause more chronic asymptomatic disease than acute severe illnesses. Such asymptomatic individuals develop anti-disease immunity and become parasite reservoirs. The workprogramme is based on a “one health” strategy capitalizing on the strength and the multidisciplinary expertise of the team members (clinicians working on the field in FG, molecular parasitology, epidemiology and immunology). The specific aims are to: 1) identify immune signatures associate to protection or pathology and 2) investigate how they are modulated by environmental factors and host multi-biome and influence the disease progression in such settings.
Data obtained should allow to identify disease phenotype biomarkers and targets for diagnosis, prognosis, prevention and treatment.
Highlights
- Partner of the Laboratory of Excellence (LabEx) “French Parasitology Alliance for Health Care” (LabEx PARAFRAP ; http://labex-parafrap.fr, 2012-2019 renewed for five years from 2020).
- Partner of the Laboratory of Excellence “Center for the study of biodiversity in Amazonia” (LabEx CEBA ; http://www.labex-ceba.fr, 2021-2019 renewed for five years from 2020).
- Flash COVID FEDER-Guiana funding obtained for 2 years (2020-2022) for the study of humoral immune responses induced by SARS-COV-2 in the Amazon context: Role in the establishment of asymptomatic disease (COVIMA).
 Project 1. Molecular and cellular host–parasite crosstalk underlying protection/ pathology during malaria (S. Pied).
Project 1. Molecular and cellular host–parasite crosstalk underlying protection/ pathology during malaria (S. Pied).
Astrocytes and M1-like microglial cells play a prominent role for in the exacerbation of neuroinflammatory processes in cerebral malaria (CM) through parasite microvesicles transferred in Astrocytes. Our workprogram is to: 1) Define mechanisms of parasite microvesicle transfer to astrocytes (coll. F. Lafont and BICELL platform at CIIL); 2) Characterize parasite microvesicles and the secretomes of glial cells (using differential metabolomics and proteomics (coll. S. Kamat, IISER Pune, India) to identify immunoregulators); 3) Examine how pro-inflammatory astrocytes and microglia interact with brain-infiltrating myeloid cells and CD8+ T cells to give rise to the neuropathology.
 Project 2. Understanding natural immunity in populations exposed to multiple protozoan infections (M. Demar, G. Prévot, F. Djossou, N. Elanga, M. Nacher, S. Pied).
Project 2. Understanding natural immunity in populations exposed to multiple protozoan infections (M. Demar, G. Prévot, F. Djossou, N. Elanga, M. Nacher, S. Pied).
We will take advantage of the high prevalence of malaria, leishmania and toxoplasmosis in the Amazonian border of French Guiana to analyze the intricate and complex mutualistic interactions between concomitant apicomplexan infections in exposed individuals. We will investigate if a concomitant chronic T. gondii and/or L. guyanensis infection in malaria patients modify the fine-tuning of the host immunity and trigger resistance to severe malarial disease. A multidisciplinary approach combining clinical field studies, molecular epidemiology, systems immunology, advanced bio-informatics, and multivariate statistical analysis will be implemented to search for functional innate and adaptive immune signatures contributing to asymptomatic disease in coinfections.
Transversal project
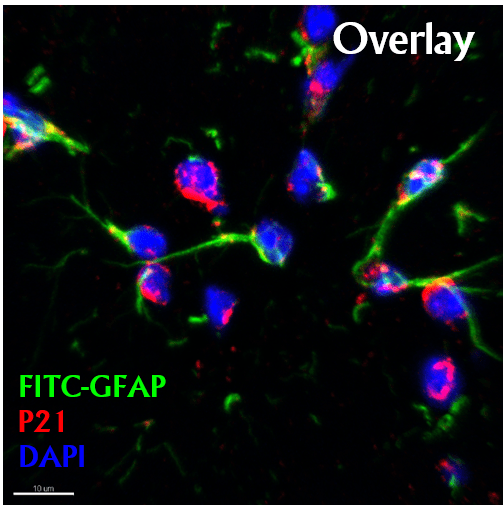
 Glial cells senescence in detrimental neurological effects associated to Malaria.
Glial cells senescence in detrimental neurological effects associated to Malaria.
The proposal will define the mechanism by which Plasmodium infection induces acute cellular senescence in astrocytes and microglial cells to promote cerebral malaria and long-lasting cognitive deficits. ─ collaboration Corrine Abbadie, CANTHER – Univ. Lille, CNRS, Inserm, CHU de Lille, Institut Pasteur de Lille – UMR9020-U1277.
Members
The team is composed of two groups: 1) group 1 at the Institute Pasteur of Lille is leaded by S. Pied DR CNRS and 2) group 2 at the University of Guiana and Cayenne Hospital (CHAR) leaded by M. Demar, PU-PH. The team is linked to the Laboratory of Parasitology–Mycology and several medical units, including the departments of 1) infectious and tropical diseases, 2) dermatology, and 3) several health centers for remote areas of Guiana. Moreover, TBIP has ties with the Clinical Investigation Center (CIC INSERM-CIE 802 leaded by M. Nacher).
Sylviane PIED
Research director CNRS, group Lille leader
ORCID number : 0000-0003-0642-1423
Magalie PIERRE DEMAR
PU-PH Univ Guyane, group Guyane leader
ORCID number : 0000-0003-3414-0821
I. LELEU
Engineer, CNRS
ORCID number : 0000-0001-8414-8982
M. GINOUVES
Engineer assistant, Univ Guyane
ORCID number : 0000-0003-2331-6588
N. SAIDI
PhD student, Univ Tunis/ Lille
F. HELLANI
PhD student, Univ Lille
J. ALLOO
PhD student, Univ Lille
K. NERON ELFORT
PhD student, Univ Guyane
G. PROSSE AHOUET
PhD student, Univ Congo
M. NACHER
PU-PH-Dir CIC CHAR, Univ Guyane
ORCID number : 0000-0001-9397-3204
F. DJOSSOU
PU-PH-Infectious diseases CHAR, Univ Guyane
ORCID number : 0000-0002-6761-0511
P. COUPPIE
PU-PH-Dermatology CHAR, Univ Guyane
ORCID number : 0000-0002-4213-2867
N. ELENGA
PU-PH CHAR, Univ Guyane
R. BLAIZOT
MCU-PH-CHAR, Univ Guyane
ORCID number : 0000-0003-3695-6824
G. PREVOT
PU, Univ Guyane
ORCID number : 0000-0002-7268-4953
P-A CAZENAVE
Professor Emeritus
ORCID number : 0000-0002-1345-8526
J. ROLAND
Researcher volunteee – CR1 CNRS
ORCID number : 0000-0003-1678-405X
M. SAOUT
Technician, Univ Guyane
Publications
Chavy A, Ferreira Dales Nava A, Luz SLB, Ramírez JD, Herrera G, Vasconcelos Dos Santos T, Ginouves M, Demar M, Prévot G, Guégan JF, de Thoisy B.
Ecological niche modelling for predicting the risk of cutaneous leishmaniasis in the Neotropical moist forest biome.
PLoS Negl Trop Dis. 2019 Aug 14;13(8):e0007629. doi: 10.1371/journal.pntd.0007629. eCollection 2019 Aug.
Blaizot R, Nabet C, Laghoe L, Faivre B, Escotte-Binet S, Djossou F, Mosnier E, Henaff F, Blanchet D, Mercier A, Dardé ML, Villena I, Demar M.
Outbreak of Amazonian Toxoplasmosis: A One Health Investigation in a Remote Amerindian Community.
Front Cell Infect Microbiol. 2020 Sep 11;10:401. doi: 10.3389/fcimb.2020.00401. eCollection 2020. PMID: 33042853.
Keswani T, Delcroix-Genete D, Herbert F, Leleu I, Lambert C, Draheim M, Salome-Desnoulez S, Saliou JM, Cazenave PA, Silvie O, Roland J, Pied S.
Plasmodium yoelii Uses a TLR3-Dependent Pathway to Achieve Mammalian Host Parasitism.
J Immunol. 2020 Dec 1;205(11):3071-3082. doi: 10.4049/jimmunol.1901317. Epub 2020 Nov 4. PMID: 33148715
Keswani T, Roland J, Herbert F, Delcroix-Genete D, Bauderlique-Le Roy H, Gaayeb L, Cazenave PA, Pied S.
Expression of CD300lf by microglia contributes to resistance to cerebral malaria by impeding the neuroinflammation.
Genes Immun. 2020 Jan;21(1):45-62. doi: 10.1038/s41435-019-0085-9. Epub 2019 Sep 10. PMID: 31501529.
Shrivastava SK, Dalko E, Delcroix-Genete D, Herbert F, Cazenave PA, Pied S.
Uptake of parasite-derived vesicles by astrocytes and microglial phagocytosis of infected erythrocytes may drive neuroinflammation in cerebral malaria.
Glia. 2017 Jan;65(1):75-92. doi: 10.1002/glia.23075. Epub 2016 Oct 3.
Keywords
Malaria ; Leishmania ; Toxoplasma ; Parasitic infection ; Co-infection ; Immune responses ; Inflammation ; Immunopathophysiology ; Immunity
Team contact
Sylviane Pied
DR CNRS, group leader
sylviane.pied@pasteur-lille.fr
03 20 87 78 02 ; 06 70 16 15 50