Efficacity and resistance to anti-tumour targeted therapies
UMR Canther CNRS 9020 – Inserm S 1277 – Lille University – CHU Lille – Institut Pasteur de Lille
Presentation
This last decade, emergence of targeted therapies profoundly changed the care of some patients displaying oncogene addiction, with improvement of their live condition or expectancy. However, all the targeted therapies have to face to emergence of resistances, leading to inevitable treatment failures. In addition, many cancers are still diagnosed at a metastatic stage, a step of the disease that escapes to treatment efficacy and still associated with a poor prognosis due to an altered response to treatment.
The global aim of our research project is to decipher the molecular mechanisms leading to oncogene addiction and later to resistance process in order to improve treatment with targeted therapies. We are focusing our attention on the lung and prostate cancers, including their metastatic progression, representing together the main causes of lethality by cancer. For both lung and prostate cancers, we take advantage on our expertise on well-documented oncogenes deregulated in these cancers including receptor tyrosine kinase (RTK) and ETS-Fusion genes. We are also developing new therapeutic approaches to correct nonsense mutations that represent 5 to 40% of the mutations affecting the tumor suppressor genes and responsible for also about 10% of any genetic diseases. We deliberately chosen to work in strong collaboration with molecular biologists, pathologists, and clinicians of the Lille university hospital who are directly involved in patient care.
Highlights
- We recently isolated from mushroom extracts the DAP (2,6 diaminopurine), a compound able to promote readthrough of mRNA harboring non-sense mutations (stop codon). Next, we search to characterize its mechanism of action. Actually, DAP is able to interfere with activity of the FTSJ1, an enzyme involved in the regulation of transfer RNA. Therefore, the compound inhibits in vivo the growth of cancer cells displaying p53 non-sense mutation, opening the way for novel strategies of antitumoral targeted therapies (Trzaska et al., Nat Commun. 2020). Press release from INSERM : https://presse.inserm.fr/un-champignon-au-secours-des-patients-atteints-de-maladies-genetiques-rares/38698/
-
Targeted therapies against MET receptor were recently approved for treatment of patients with lung cancers displaying MET mutations. Nevertheless, these patients display resistances limiting therapies efficacy. Based on tumor samples from patients treated at Lille and through resistance reconstitution in cell lines, we identified a novel mechanism of resistance involving the reactivation of the PI3K pathway. This study opens the way for co-treatment strategies to counteract resistances (Jamme et al, J Thorac Oncol 2020).
- We previously demonstrated the MET is a dependence receptor able to induce either cell survival in presence of ligand or cell death in its absence. Thanks to generation knock-in mice, we demonstrated this year that this dual function is important for regulation of the survival/apoptosis balance in an organism and notably in liver. This original MET function could be important also for tumorigenesis (Duplaquet et al, ELife, 2020). Press release from CNRS : https://insb.cnrs.fr/fr/cnrsinfo/le-recepteur-met-une-des-clefs-de-lequilibre-des-forces-entre-vie-et-mort-cellulaire


Transversal project
 COVID-19
COVID-19
The Target team is also involved in the fight against COVID-19 (ARC Flash COVID, Martine Duterque-Coquillaud). The objective of the project is to evaluate the influence of androgens during SARS-CoV-2 infection. in the viral infection. Indeed, expression of some virus receptors are dependent of androgens in organs such as prostate. This project is performed in collaboration with the team of Dr François Trottein (Centre d’Infection et d’Immunité de Lille, IPL) and CHU Lille. This project could lead to a better understanding of severe COVID-19.

Members
David TULASNE
DR2 Inserm, responsable de l’équipe
Numéro ORCID : 0000-0002-6764-7242
Martine DUTERQUE-COQUILLAUD
DR2 CNRS
Numéro ORCID : 0000-0003-3943-5629
Anne CHOTTEAU-LELIEVRE
PR2 Univ Lille
Zoulika KHERROUCHE
CR1 IPL
Numéro ORCID : 0000-0003-0666-5698
Marie-José TRUONG
Chercheur IPL
Numéro ORCID : 0000-0001-6697-6266
Fabrice LEJEUNE
CRCN Inserm
Numéro ORCID : 0000-0002-5132-3585
Marie-Hélène VIEILLARD
Onco-Rhumatologue, COL et CHU Lille
Sarah HUMEZ
Anatomo-Pathologiste, CHU Lille
Arnauld VILLERS
Urologue – PU-PH, CHU Lille
Numéro ORCID : 0000-0002-6298-7758
Xavier LEROY
Anatomo-Pathologiste – PU-PH, CHU Lille
Alexis CORTOT
Pneumologue – PU-PH, CHU Lille
Simon BALDACCI
Pneumologue, CHU Lille
Catherine AMPEN-GUFFROY
IE CNRS
Virginie POIX
IE CNRS, CDD
David HANNEBIQUE
AI CNRS (Animal facility)
Anne-Claire FLOURENS
Technician Inserm
Nathalie VANPOUILLE
Technician IPL
Isabelle DAMOUR
Technician IPL
Audrey VINCHENT
Technician IPL
Marie FERNANDES
PhD student, Univ Lille
Anthony TURPIN
PhD student, Univ Lille – Oncologist, CHU Lille
Martine PALMA
PhD student, Univ Lille
Jonathan OLIVIER
PhD student, Univ Lille – Urologist, CHU Lille
Célia GUERIN
PhD student, Univ Lille
Elisa CAROUGE
PhD student, Univ Lille
Publications
Duplaquet L, Leroy C, Vinchent A, Paget S, Lefebvre J, Vanden Abeele F, Lancel S, Giffard F, Bidaux G, Heliot L, Poulain L, Furlan A and Tulasne D.
Control of cell death/survival balance by the MET dependence receptor.
Elife. 2020 Feb 24;9. IF 7.551.
Jamme P, Fernandes M, Copin MC, Descarpentries C, Escande F, Morabito A, Grégoire V, Jamme M, Baldacci S, Tulasne D, Kherrouche Z, and Cortot AB.
Alterations in the PI3K pathway drive resistance to MET inhibitors in NSCLC harboring MET exon 14 skipping mutations. Journal of Thoracic Oncology.
2020 Mar 10. IF 12.460.
Trzaska C, Amand S, Bailly C, Leroy C, Marchand V, Duvernois-Berthet E, Saliou JM, Benhabiles H, Werkmeister E, Chassat T, Guilbert R, Hannebique D, Mouray A, Neu-Yilik G, Copin MC, Moreau PA, Prévotat A, Reix P, Hubert D, Gérardin M, Adriaenssens E, Hentze M, Kulozik A, Westhof E, Tulasne D, Motorin Y, Rebuffat S and Lejeune F.
2,6-Diaminopurine as a highly potent corrector of UGA nonsense mutations.
Nat Commun. 2020 Mar 20;11(1):1509. IF 11.878.
Baldacci S, Figeac M, Antoine M, Descarpentries C, Kherrouche Z, Jamme P, Copin MC, Tulasne D, Nanni I, Beau-Faller M, Melaabi S, Levallet G, Quoix E, Moro-Sibilot D, Friard S, Missy P, Barlesi F, Cadranel J, and Cortot AB.
High MET overexpression does not predict the presence of MET exon 14 splice mutations in NSCLC : results from the IFCT Predict.amm study. Journal of Thoracic Oncology.
2020 Jan;15(1):120-124. IF 12.460.
[Delliaux C, Tian TV], Bouchet M, Fradet A, Vanpouille N, Flourens A, Deplus R, Villers A, Leroy X, Clézardin P, de Launoit Y, Bonnelye E, Duterque-Coquillaud M.
TMPRSS2:ERG gene fusion expression regulates bone markers and enhances the osteoblastic phenotype of prostate cancer bone metastases.
Cancer Lett. 2018 Dec 1;438:32-43. IF 6.508.
Keywords
Cancer ; Lung ; Prostate ; MET Receptor ; ETS Transcription factors ; Transcriptional regulation ; mRNA quality control ; Receptor tyrosine kinase ; Intracellular signaling ; Tumor suppressor genes ; Survival/apoptosis balance ; Transcriptional regulation ; lung Cancer ; Targeted therapies ; Resistance ; Prostate cancer, Bone metastasis, ETS fusion, Transcriptional regulation ; ETS transcription factors ; MET Receptor ; Transcriptionnal regulation ; Murine model of breast cancer ; tumoral microenvironment ; immune cells ; tumor-infiltrating lymphocytes (TILs) ; nonsense mutations ; nonsense-mediated mRNA decay (NMD) ; readthrough ; targeted therapeutic approaches ; screening ; genetic diseases
Team contact
David Tulasne
Group leader
david.tulasne@ibl.cnrs.fr
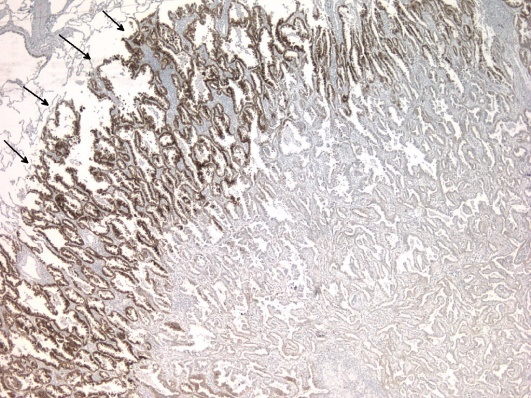
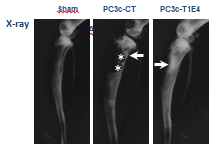
Lung tumor expressing MET receptor at the invasion front.
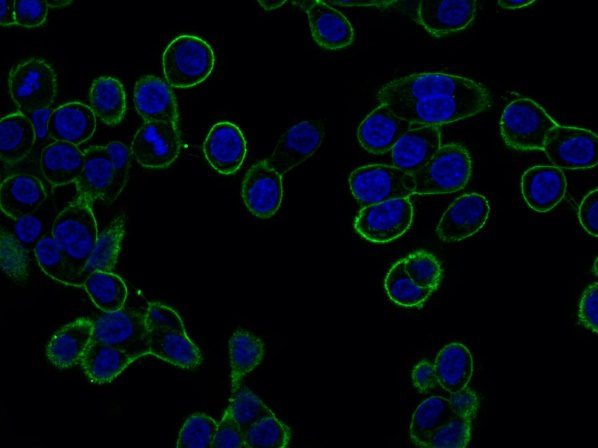
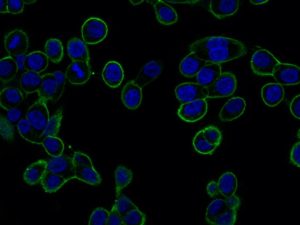
GTL16 cancer cells expressing MET receptor at plasma membrane (green staining).
Colocalization in cytoplasmic foci in HeLa cells of UPF1 (in green) and DCP1a (in red) upon colchicine treatment.